Danocrine (danazol) Steroid Profile
Table of Contents
- Danocrine (danazol)
- What Is Danocrine Use?
- How to use Danocrine Capsule
- How Danocrine works
- What Are the Side Effects of Danocrine?
- Indications
- Dosage for Danocrine
- What Drugs, Substances, or Supplements Interact with Danocrine?
- Half-life
- Danocrine During Pregnancy or Breastfeeding
- Warnings
- Before taking this medicine
- References
Danocrine (danazol) is a synthetic steroid derived from ethisterone that has a structure similar to testosterone, an androgen or male sex hormone. Discovered in 1963, it has been in medical use since 1971. A synthetic steroid with anti-gonadotropic and anti-estrogenic activities that acts as an anterior pituitary suppressant by inhibiting the pituitary output of gonadotropins. It possesses some androgenic properties. Danazol has been used in the treatment of endometriosis and some benign breast disorders.
Danocrine (danazol)

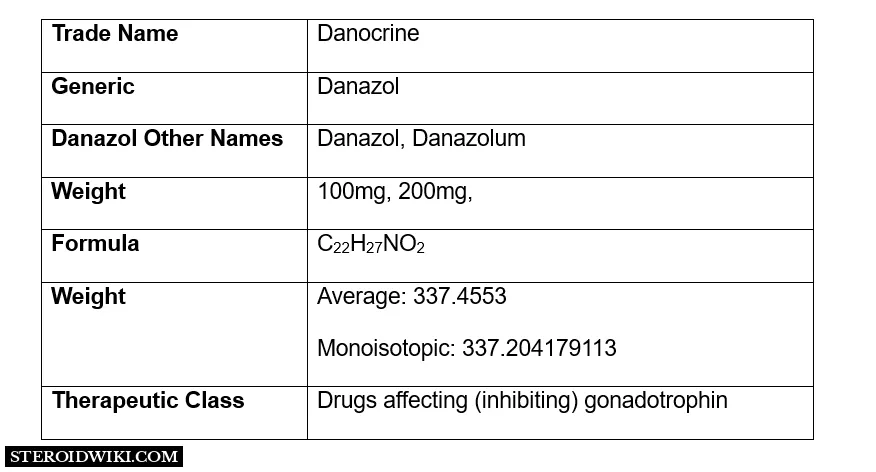
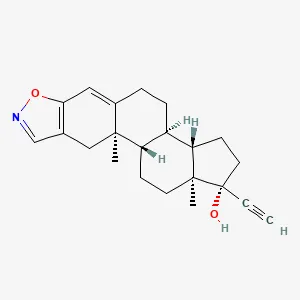
DANOCRINE, a brand of danazol, is a synthetic steroid derived from ethisterone. It is a white to pale yellow crystalline powder, practically insoluble or insoluble in water, and sparingly soluble in alcohol. Chemically, danazol is 17α-Pregna-2,4-dien-20-yno [2,3-d]isoxazol-17-ol. The molecular formula is C22H27NO2. It has a molecular weight of 337.46 and the following structural formula:
DANAZOL Structural Formula
Danocrine capsules for oral administration contain 50 mg, 100 mg or 200 mg of danazol.
What Is Danocrine Use?
Danocrine (danazol) is a steroid used to treat endometriosis and fibrocystic breast disease. It is also used to prevent attacks of angioedema in people with an inherited form of this disorder. This medication is used in women to treat pelvic pain and infertility due to a certain disorder (endometriosis) and also to treat breast pain/tenderness/nodules due to a certain breast condition (fibrocystic breast disease). It is also used in both men and women to prevent swelling of the abdomen/arms/legs/face/airway due to a certain congenital disease (hereditary angioedema). Danazol is an androgen similar to testosterone. For the treatment of endometriosis and fibrocystic breast disease, it works by decreasing the amount of hormones made by the ovaries. These hormones usually make the conditions worse. For the treatment of angioedema, danazol helps to increase the amount of a certain protein in your body's defence system (immune system).
How to use Danocrine Capsule
Take this medication by mouth as directed by your doctor, usually twice daily. You may take this medication with or without food, but it is important to choose one way and take this medication the same way with every dose.
How Danocrine works
Danocrine mainly works by suppressing hormone production by the pituitary and sex glands (gonads), which are responsible for sexual development and reproduction. Danocrine has multiple effects on the body, including many androgenic side effects.
In hereditary angioedema, a rare genetic disorder marked by insufficient blood levels of the C1 esterase inhibitor (C1INH) protein and C4 protein, Danocrine helps to prevent spontaneous edema attacks by increasing C1INH and C4 levels in the blood. Exactly how it regulates these protein levels is not fully understood.
What Are the Side Effects of Danocrine?
Danocrine may cause serious side effects including:
- Hives
- Difficulty breathing
- Swelling of your face, lips, tongue, or throat
- Loss of appetite
- Stomach pain (upper right side)
- Cough with bloody mucus
- Vomit that looks like coffee grounds
- Yellowing of the skin or eyes (jaundice)
- Bloody or tarry stools
- Dark urine
- Swelling or weight gain
- Hoarse or deepened voice
- Sore throat
- Hair loss
- Increased hair growth
- Acne or other skin problems
- Unexplained muscle pain
- Tenderness
- Weakness
- Severe headache
- Ringing in your ears
- Dizziness
- Nausea
- Vision problems
- Pain behind your eyes
- Sudden numbness or weakness
- Problems with vision or speech, and
- Swelling or redness in arm or leg
Get medical help right away, if you have any of the symptoms listed above.
Common side effects of Danocrine (danazol) include
- Acne or other skin problems
- Increased hair growth or hair loss
- Weight gain
- Breast changes
- Deepened voice
- Hoarseness
- Sore throat
- Nervousness
- Increased sweating
- Flushing (warmth, redness, or tingly feeling under your skin
- Decreased amount of semen released during sex
- Changes in your menstrual periods
- Unusual vaginal bleeding or spotting, or vaginal dryness/discomfort/itching
Seek medical care if you have the following serious side effects:
Serious symptoms
Serious eye symptoms
- Sudden vision loss
- Blurred vision
- Tunnel vision
- Eye pain or swelling
- Seeing halos around lights
Serious heart symptoms
- Fast, irregular, or pounding heartbeats
- Fluttering in your chest
- Shortness of breath
- Sudden dizziness
- Lightheadedness
- Passing out
Severe headache
- Confusion
- Slurred speech
- Arm or leg weakness
- Trouble walking
- Loss of coordination
- Feeling unsteady
- Very stiff muscles
- High fever
- Profuse sweating
- Tremors
Indications
Endometriosis
Danocrine is indicated for the treatment of endometriosis amenable to hormonal management.
Fibrocystic Breast Disease
Most cases of symptomatic fibrocystic breast disease may be treated by simple measures (e.g., padded brassieres and analgesics).
In infrequent patients, symptoms of pain and tenderness may be severe enough to warrant treatment by suppression of ovarian function. Danocrine is usually effective in decreasing nodularity, pain, and tenderness. It should be stressed to the patient that this treatment is not innocuous in that it involves considerable alterations of hormone levels and that recurrence of symptoms is very common after cessation of therapy.
Hereditary Angioedema
Danocrine is indicated for the prevention of attacks of angioedema of all types (cutaneous, abdominal, laryngeal) in males and females.
Dosage for Danocrine
Usual Adult Dose for Endometriosis:
Mild Disease
Initial dose: 200 to 400 mg orally per day, given in 2 divided doses
Maintenance dose: Gradual downward titrations should be performed to maintain amenorrhea.
Duration of therapy: Up to 9 months
Moderate to Severe Disease or Patients Infertile due to Endometriosis:
Initial dose: 800 mg orally per day, given in 2 divided doses
Maintenance dose: Gradual downward titrations should be performed to maintain amenorrhea.
Duration of therapy: Up to 9 months
Things to keep in mind
- Treatment should begin during menstruation, OR appropriate pregnancy tests should be performed before starting treatment to ensure that the patient is not pregnant while on treatment.
- Treatment should continue uninterrupted 3 to 6 months, but may continue for up to 9 months.
- Treatment may be reinstituted if symptoms recur.
Use: Treatment of endometriosis amenable to hormonal management
Usual Adult Dose for Fibrocystic Breast Disease
100 to 400 mg orally per day, given in 2 divided doses
Things to keep in mind
- Most patients may be treated with simple measures (e.g., padded brassieres, analgesics). Patients requiring treatment with this drug may have symptoms of severe pain and tenderness.
- Patients should be advised that treatment is not innocuous; hormone level alterations and symptom recurrence are very common after discontinuation of treatment.
- This drug is usually effective in decreasing symptoms of fibrocystic breast disease (e.g., nodularity, pain, tenderness); pain and tenderness are typically eliminated in 2 to 3 months, and nodularity is usually eliminated after 4 to 6 months of uninterrupted treatment.
- Approximately 50% of patients will have symptom recurrence within 1 year; treatment may be reinstated in this patient population if necessary.
What Drugs, Substances, or Supplements Interact with Danocrine?
Danocrine may interact with blood thinners or carbamazepine. Tell your doctor about all medications and supplements you use. Danocrine can cause birth defects.
Half-life
Danocrine has a half-life of approximately 24 hours.
Danocrine During Pregnancy or Breastfeeding
Do not use Danocrine if you are pregnant. Tell your doctor if you become pregnant during treatment. Before you start taking Danocrine, you may need to have a pregnancy test to make sure you are not pregnant. Use an effective barrier form of birth control (such as a condom or diaphragm with spermicide gel or inserts). Hormonal contraception (such as birth control pills, injections, implants, skin patches, and vaginal rings) may not be effective to prevent pregnancy during treatment. Breastfeeding is not recommended while using this drug.
Warnings
You should not use danazol if you have: undiagnosed vaginal bleeding, porphyria(a disorder that affects how your body makes red blood cells (RBC), severe liver or kidney disease, severe heart problems, or if you have ever had a stroke or blood clot, or cancer of the breast, uterus/cervix, or vagina.
Do not use danazol if you are pregnant
Use effective birth control, and tell your doctor if you become pregnant.
Do not breastfeed while using danazol.
Before taking this medicine
You should not use danazol if you are allergic to it, or if you have:
- undiagnosed vaginal bleeding;
- severe heart problems;
- a history of stroke or blood clot;
- severe liver or kidney disease;
- porphyria (a genetic enzyme disorder that causes symptoms affecting the skin or nervous system); or
- a history of hormone-related cancer, or cancer of the breast, uterus/cervix, or vagina.
- To make sure danazol is safe for you, tell your doctor if you have ever had:
- heart problems;
- high blood pressure;
- liver disease;
- kidney disease;
- epilepsy or other seizure disorder;
- diabetes; or
- migraine headaches.
You may need to have a negative pregnancy test before starting this treatment.
References
- Devalapally, H., Silchenko, S., Zhou, F., McDade, J., Goloverda, G., Owen, A. and Hidalgo, I.J., 2013. Evaluation of a nanoemulsion formulation strategy for oral bioavailability enhancement of danazol in rats and dogs. Journal of Pharmaceutical Sciences, 102(10), pp.3808-3815.
- Danocrine Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing WebMD. WebMD. Available at: https://www.webmd.com/drugs/2/drug-1306/danocrine-oral/details (Accessed: 30 January 2024).
- Badawy, S.I.F., Ghorab, M.M. and Adeyeye, C.M., 1996. Characterization and bioavailability of danazol-hydroxypropyl β-cyclodextrin coprecipitates. International journal of pharmaceutics, 128(1-2), pp.45-54.
- Dmowski, W.P., 1990. Danazol. A synthetic steroid with diverse biologic effects. The Journal of reproductive medicine, 35(1 Suppl), pp.69-74.
- Elsevier, Inc. Danazol (Danocrine) Capsules: Uses & Side Effects, Cleveland Clinic. Available at: https://my.clevelandclinic.org/health/drugs/18343-danazol-capsules (Accessed: 30 January 2024).