Tirzepatide: Complete Profile, Dosage, Mechanism of Action, Advantages and Disadvantages
Table of Content
- What is Tirzepatide?
- Tirzepatide – Mechanism of Action
- Is Tirzepatide used for weight loss?
- How does tirzepatide cause weight loss?
- Important information
- Before using Tirzepatide
- How should I use Tirzepatide?
- Dosage
- What should I do if I miss a dose?
- What are the side effects of Tirzepatide?
- Interactions
- Storage
- What are the ingredients in tirzepatide?
- The bottom line
- References
Brand name: Mounjaro
Dosage form: Subcutaneous injection
Drug class: Incretin mimetics
What is Tirzepatide?

Tirzepatide is an injectable dual agonist for the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. Compared to GLP-1, GIP is regarded as the primary insulinotropic hormone and has been shown to play a more significant function in postprandial insulin production. Tirzepatide has also been found to be effective in weight reduction, although it is not yet an FDA-approved weight loss medication. Tirzepatide stimulates the GIP and GLP-1 receptors. Tirzepatide is exclusively for adults and should be taken with a healthy diet and regular exercise.
Diabetes is a long-term disorder in which your blood sugars (HbA1c) grow abnormally high due to your body's inability to generate or use insulin correctly. Over time, excessive blood sugar levels can lead to major health complications such as kidney disease, heart disease, eyesight loss, and blood circulation disorders.
Tirzepatide reduces blood sugar levels by boosting insulin production and decreasing the amount of sugar produced by the liver. It also slows the rate at which food flows through your body, making you feel fuller for longer, which may aid with weight reduction. Tirzepatide is a GIP/GLP-1 receptor agonist, stimulating the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. Tirzepatide can be used alone or with other diabetic medications such as sulfonylureas, SGLT2 inhibitors, or metformin.
Tirzepatide – Mechanism of Action
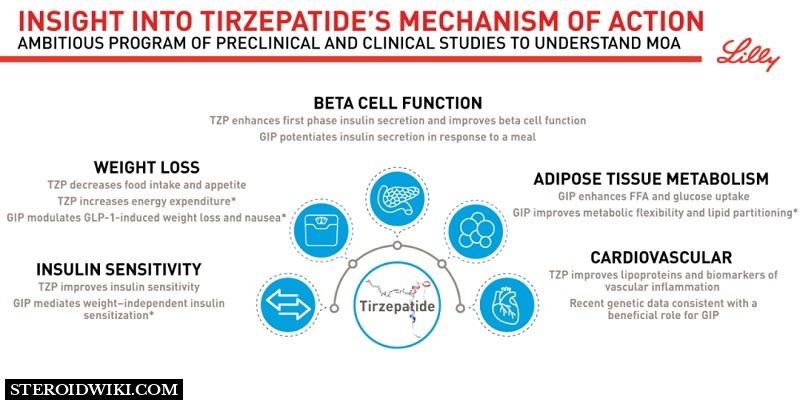
Tirzepatide is a glucagon-like peptide-1 (GLP-1) receptor agonist and a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist. It binds to and activates the GIP and GLP-1 receptors, which are the targets of endogenous GIP and GLP-1 hormones. GIP and GIP-1 are hormones that are largely secreted by the body's small intestine and help increase insulin release after eating. Tirzepatide belongs to a family of medications known as incretin mimetics, and both hormones are known as incretins. Tirzepatide regulates blood sugar by directly stimulating the GIP and GLP-1 pathways.
It is unusual because it is the first drug to combine two classes: a glucagon-like peptide-1 (GLP-1) receptor agonist and a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist. As a result, Tirzepatide is classified as a dual GLP-1/GIP receptor agonist.
Both GLP-1 and GIP are increasing hormones, which are hormones produced after eating. Tirzepatide mimics the effects of several drugs. This involves instructing the pancreas to release insulin after eating and instructing the liver to reduce the amount of glucose it produces. These effects aid in feeling full, slowing digestion, and lowering blood sugar.
Is Tirzepatide used for weight loss?
Tirzepatide has been found to help overweight people lose weight. Phase 3 clinical studies for weight loss in individuals who are overweight, obese, or have weight-related health concerns are now underway.
The average weight reduction with tirzepatide after 72 weeks in the SURMOUNT-1 clinical study was 15% for the 5mg dosage, 19.5% for the 10mg dose, and 20.9% for the 15mg dose. Tirzepatide is not an FDA-approved weight reduction medicine; it is approved to assist in lowering blood sugar levels in type 2 diabetes patients and should be taken with diet and exercise.
How does tirzepatide cause weight loss?
In addition to helping lower blood sugar, tirzepatide helps with weight loss in a few ways:
- It signals to the brain that you're full, so you eat less.
- It slows food movement through your stomach so you feel full longer.
Important information
Tirzepatide has been linked to thyroid C-cell tumors. It is critical to inform your doctor if you or someone in your family has a history of thyroid cancer or a disease known as Multiple Endocrine Neoplasia (MEN) syndrome. This is a sickness that causes tumors in the endocrine glands to form. If you have a lump or swelling in your neck, trouble swallowing, hoarseness, or shortness of breath, you should contact your doctor once.
Remember to attend your doctor's healthcare professional. Your doctor may want to do some tests to check how the medicine works in your body.
Before using Tirzepatide
If you are allergic to Tirzepatide, other drugs, or any of the chemicals in this medication, tell your doctor and chemist immediately. A list of the components of this drug may be found at the bottom of this page.
- Inform your doctor if you have had kidney or pancreatic disease, a history of diabetic retinopathy, or any stomach issues, such as difficulties digesting meals.
- Inform your doctor if you want to get pregnant, if you are pregnant, or breastfeeding. If you become pregnant while using this medication, notify your doctor immediately.
How should I use Tirzepatide?

You should carefully read the recommendations on your prescription label and ask your doctor to explain any section you don't understand. It is critical to take this medication exactly as advised. Do not take more or less of it or more frequently than your doctor has suggested.
Tirzepatide is available as a solution (liquid) in a pre-filled pen injected subcutaneously (under the skin).
- It is normally administered once a week.
- It may be taken with or without food at any time of the day.
- Injections can be given into the thigh, belly, or upper arm.
- With each dosage, alternate injection locations.
Your doctor will most likely begin you on a modest dose that will be progressively raised but no more than once every four weeks. You may modify the day of the week you take tirzepatide as long as at least three days pass between doses.
You can deliver insulin in the same region as tirzepatide, but not exactly next to each other. Insulin and tirzepatide should be administered separately and not in the same injection.
Tirzepatide manages but does not cure type 2 diabetes. It may take four weeks or more to see the full effect of this medication. Even if you feel OK, keep taking this medication. Do not discontinue using this medication without first seeing your doctor.
Dosage
Initial dose
2.5 mg subcutaneously once a week
After four weeks: The dosage should be increased to 5 mg subcutaneously once a week.
For additional glycemic control: Increase dosage in 2.5 mg increments after at least four weeks on the current dose.
Maximum dose
15 mg subcutaneously once a week
What should I do if I miss a dose?
If you miss a Tirzepatide dosage, take it as soon as you recall if it is within four days after the missing dose. If it has been more than four days since your last dosage, you should skip the missed dose and resume your usual dosing plan. Do not provide two dosages within three days of each other.
What are the side effects of Tirzepatide?
Tirzepatide may cause side effects. You should tell your doctor if any of these symptoms are severe or do not go away:
- vomiting
- nausea
- diarrhea
- decreased appetite
- constipation
- upset stomach
Tirzepatide may induce hypoglycemia (low blood sugar). Dizziness or lightheadedness, blurred vision, anxiety, irritability or mood swings, sweating, slurred speech, hunger, confusion or sleepiness, shakiness, weakness, headache, high heart rate, and feeling nervous and uneasy are all symptoms and indicators of low blood sugar. There are several potentially dangerous adverse effects associated with tirzepatide. These are some examples:
- A severe allergic response has occurred. This might result in a rash and difficulty breathing, requiring quick medical intervention.
- Pancreatitis. This can result in severe stomach or back discomfort, vomiting, and fevers, requiring rapid medical intervention.
- Cancer of the thyroid: If you or a close family member has a history of certain forms of thyroid cancer, your doctor may advise you to avoid tirzepatide.
- Gallbladder problems: This can include gallstones.
- Low blood sugar (hypoglycemia): This is especially possible when used with insulin, sulfonylureas, or glinides.
Interactions
Inform your doctor and chemist about any prescription or over-the-counter drugs, vitamins, nutritional supplements, or herbal items you intend to use or are now taking. They may interact with tirzepatide, and your doctor may need to adjust your prescription doses or closely monitor you for adverse effects. While taking this medication, birth control pills may not function as well. Your doctor may advise you to use a different method of birth control for four weeks after starting tirzepatide and for four weeks after any dose adjustment.
Combining tirzepatide with other diabetic medications, such as sulfonylureas or insulin, may raise your risk of hypoglycemia. Discuss low blood sugar and how to treat it with your doctor. Many other drugs may interact with Mounjaro, including prescription and over-the-counter medicines, vitamins, and herbal products.
Storage
The Mounjaro brand of tirzepatide should be kept in the refrigerator at temperatures ranging from 36°F to 46°F (2°C to 8°C). To protect it from light, keep it in its original box until usage. Single-dose pens can be kept at room temperature for 21 days if necessary. Only store your medicine as recommended. Make certain you understand how to keep your medication properly.
What are the ingredients in tirzepatide?
Mounjaro Active ingredient: tirzepatide
Mounjaro Inactive ingredients:
- Sodium chloride
- Sodium phosphate
- Dibasic heptahydrate,
- Water for injection.
Hydrochloric acid and sodium hydroxide solutions may have been added to adjust the pH.
The bottom line
Tirzepatide is a weekly injectable that can help persons with Type 2 diabetes decrease their blood sugar levels. However, it also shows encouraging outcomes in studies for those who are obese or overweight but do not have diabetes. Tirzepatide, when combined with diet and exercise, can result in considerable weight loss – up to 20%. The FDA has not yet authorized it for weight reduction, but it may be shortly. The FDA has awarded tirzepatide Fast Track status for expediting a prospective weight reduction approval.
Tizepatide tells your brain that you're full, slows digestion, and decreases blood sugar levels. It might be one of several weight-loss strategies. However, it may not be suitable for everyone. Be sure to talk with your healthcare provider about the best option.
Read more on HGH and Peptides.
References
- Bailey, C.J., 2021. Tirzepatide: a new low for bodyweight and blood glucose. The Lancet Diabetes & Endocrinology, 9(10), pp.646-648.
- Ebell, M.H., 2023. Tirzepatide Helps Adults with Obesity without Diabetes Lose 15% to 21% of Their Body Weight over 72 Weeks. American Family Physician, 107(1), pp.99-99.
- Jastreboff, A.M., Aronne, L.J., Ahmad, N.N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M.C. and Stefanski, A., 2022. Tirzepatide once weekly for the treatment of obesity. New England Journal of Medicine, 387(3), pp.205-216.
- Tirzepatide plan MinuteMD Telemedicine. Available at: https://www.minutemd.com/product/tirzepatide/?v=3e8d115eb4b3
- Does tirzepatide work for weight loss, and is it safe? GoodRx. Available at: https://www.goodrx.com/conditions/weight-loss/tirzepatide-and-weight-loss
- Bailey, C.J., 2021. Tirzepatide: a new low for bodyweight and blood glucose. The Lancet Diabetes & Endocrinology, 9(10), pp.646-648.
Disclaimer: SteroidWiki doesn't promote unlawful usage of any chemical compound. The knowledge presented in this article and on the website is purely for educational purposes. If you intend to use the information provided in this article for any purpose, please make sure to check & comply with the laws of your country or area.