Testopel (testosterone pellets) steroid profile
Table of Contents
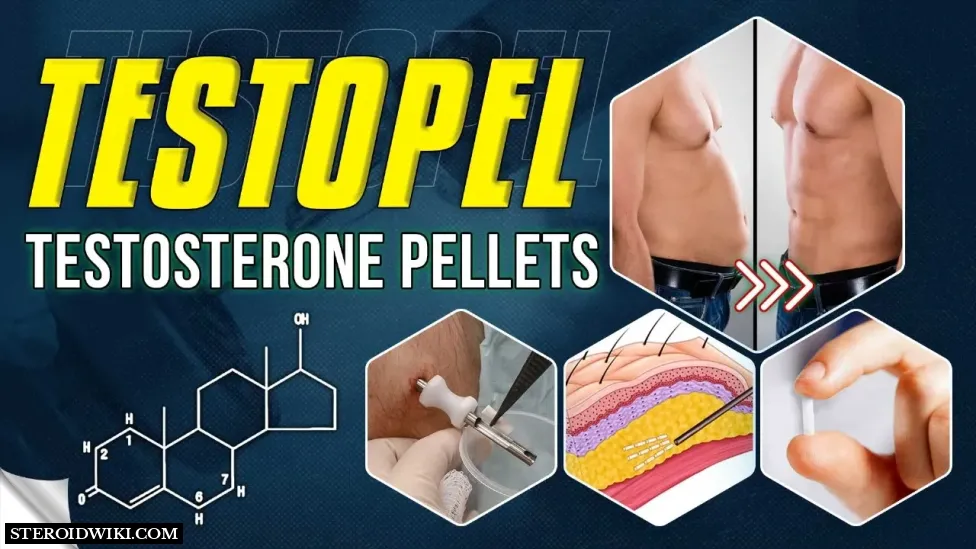
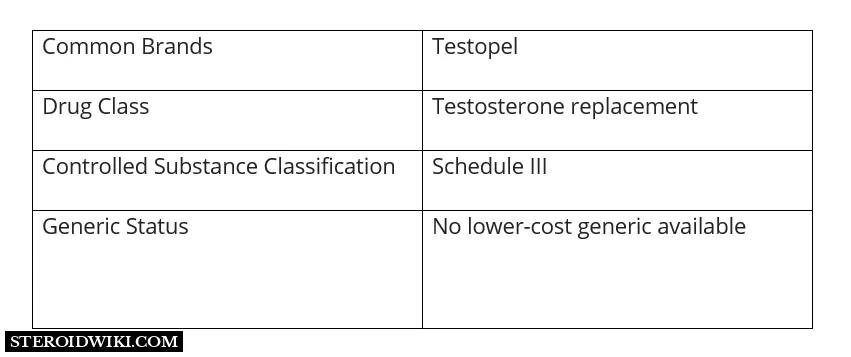
What Is Testopel?
Testopel (testosterone pellets), the male sex hormone, is a steroid used for testosterone replacement therapy in adult males for conditions associated with low or absent testosterone levels in the body, such as primary hypogonadism (congenital or acquired), hypogonadotropic hypogonadism (congenital or acquired), and to stimulate puberty in carefully selected males with clearly delayed puberty.
Testopel (testosterone pellets) are cylindrical, 3.2mm (1/8 inch) in diameter and around 9mm long. Each sterile pellet weighs around 78mg (75mg testosterone) and is ready to implant.
Androgens are steroids that cause and sustain primary and secondary male sex characteristics. This class of hormones includes testosterone.
The structural formula for testosterone follows:
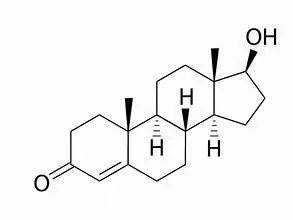
Testopel (testosterone) pellets working
Testopel (testosterone) pellets are a testosterone replacement that mimics the natural sex hormone. Testosterone is responsible for the development and maintenance of several masculine characteristics. Testopel (testosterone) pellets restore normal and healthy testosterone levels in the body.
Dosage for Testopel
The recommended dosage for Testopel testosterone pellets for replacement treatment in androgen-deficient males is 150mg to 450mg subcutaneously every 3 to 6 months.
Ingredients
Each testopel (testosterone pellet) for subcutaneous implantation has 75mg of testosterone. In addition, each pellet includes the following inactive ingredients: stearic acid NF 0.97mg and polyvinylpyrrolidone USP 2 mg.
testopel (testosterone pellets) contain crystalline testosterone. When implanted subcutaneously, the pellets gradually release the hormone, producing a long-acting androgenic effect.
Uses for Testopel
Testopel (testosterone) pellets are used for
- Low testosterone (hypogonadism)
- Stimulate puberty in certain males with delayed puberty
Males
Androgens are indicated for replacement therapy in conditions associated with a deficiency or absence of endogenous testosterone.
- Primary hypogonadism (congenital or acquired) - testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testes syndrome; or orchiectomy.
- Hypogonadotropic hypogonadism (congenital or acquired) - gonadotropic LHRH deficiency, or pituitary - hypothalamic injury from tumors, trauma, or radiation.
If the above conditions occur before puberty, androgen replacement therapy will be needed during the adolescent years for the development of secondary sex characteristics. Prolonged androgen treatment will be required to maintain sexual characteristics in these and other males who develop testosterone deficiency after puberty. - Androgens can be utilized to induce puberty in carefully chosen males with clearly delayed puberty. These individuals often have a family history of delayed puberty that is not caused by a pathological condition; puberty is predicted to occur naturally at a somewhat late age. If psychological assistance does not work for these people, brief therapy with modest dosages may be warranted. Before administering androgens, address the potential unfavorable effect on bone maturation with the patient and parents. Every 6 months, an x-ray of the hand and wrist should be performed to measure bone age and check the effect of therapy on epiphyseal centers.
Dosage for Testopel
The suggested dosage for androgens varies depending on the age, and diagnosis of the individual patient. Dosage is adjusted according to the patient’s response and the appearance of adverse reactions. The dosage guideline for the testosterone pellets for replacement therapy in androgen-deficient males is 150mg to 450mg subcutaneously every 3 to 6 months. Various dosage regimens have been used to induce pubertal changes in hypogonadal male.
Some experts have advocated lower doses initially, gradually increasing the dose as puberty progresses, with or without a decrease in maintenance levels. Other experts emphasize that higher dosages are needed to induce pubertal changes and lower dosages can be used for maintenance after puberty. The chronological and skeletal ages must be taken into consideration, both in determining the initial dose and in adjusting the dose.
Dosages in delayed puberty generally are in the lower range of that listed above and, for a limited duration, for example, 4 to 6 months. The number of pellets to be implanted depends upon the minimal daily requirements of testosterone propionate determined by a gradual reduction of the amount administered parenterally.
The usual dosage is as follows:
Implant two 75mg pellets for each 25mg testosterone propionate required weekly. Thus when a patient requires injections of 75mg per week, it is usually necessary to implant 450mg (6 pellets). With injections of 50mg per week, implantation of 300mg (4 pellets) may suffice for approximately three months. With lower requirements by injection, correspondingly lower amounts may be implanted. It has been found that approximately one-third of the material is absorbed in the first month, one-fourth in the second month and one-sixth in the third month. Adequate effect of the pellets ordinarily continues for three to four months, sometimes as long as six months.
Side Effects for Testopel
Common side effects of Testopel include:
- More erections than normal or erections that last a long time
- Nausea
- Vomiting
- Changes in skin color
- Ankle swelling
- Changes in body hair
- Male pattern baldness
- Acne
- Suppression of certain clotting factors
- Bleeding in patients on blood thinners
- Increase or decrease in libido (sex drive)
- Headache
- Anxiety
- Depression
- Inflammation and pain at the implantation site, and rarely
- A serious allergic reaction (anaphylaxis)
Serious side effects:
- Serious eye symptoms such as sudden vision loss, blurred vision, tunnel vision, eye pain or swelling, or seeing halos around lights;
- Serious heart symptoms such as fast, irregular, or pounding heartbeats; fluttering in your chest; shortness of breath; and sudden dizziness, lightheadedness, or passing out;
- Severe headache, confusion, slurred speech, arm or leg weakness, trouble walking, loss of coordination, feeling unsteady, very stiff muscles, high fever, profuse sweating, or tremors.
The following adverse reactions have been identified during post-approval use of testosterone replacement therapy, including TESTOPEL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Implantation Site Infection and Pellet Extrusion
Endocrine and Urogenital, Male.
Gynecomastia and excessive frequency and duration of penile erections. Oligospermia may occur at high dosages.
Skin and Appendages
Hirsutism, male pattern of baldness, and acne.
Cardiovascular Disorders
Myocardial infarction, stroke.
Fluid and Electrolyte Disturbances
Retention of sodium, chloride, water, potassium, calcium, and inorganic phosphates.
Gastrointestinal
Nausea, cholestatic jaundice, alterations in liver function tests, rarely hepatocellular neoplasms, and peliosis hepatis.
Hematologic
Suppression of clotting factors II, V, VII, and X, bleeding in patients on concomitant anticoagulant therapy, and polycythemia.
Nervous System
Increased or decreased libido, headache, anxiety, depression, and generalized paresthesia.
Metabolic
Increased serum cholesterol.
Vascular Disorders
Venous thromboembolism
Miscellaneous
Rarely anaphylactoid reactions.
Drug Interactions for Testopel
Testopel may interact with anticoagulants, oxyphenbutazone, and insulin. Tell your doctor about all medications and supplements you use.
- Anticoagulants. C-17 substituted derivatives of testosterone, such as methandrostenolone have been reported to decrease the anticoagulant requirements of patients receiving oral anticoagulants. Patients receiving oral anticoagulant therapy require close monitoring, especially when androgens are started or stopped.
- Oxyphenbutazone. Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone.
- Insulin. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and insulin requirements.
Drug/Laboratory Test Interferences
Androgens may decrease levels of thyroxine-binding globulin, resulting in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Testopel During Pregnancy or Breastfeeding
Testopel is not indicated for use in women, therefore it is unlikely to be used during pregnancy or while breastfeeding.
References
- McCullough, A.R., Khera, M., Goldstein, I., Hellstrom, W.J., Morgentaler, A. and Levine, L.A., 2012. A multi-institutional observational study of testosterone levels after testosterone pellet (Testopel®) insertion. The journal of sexual medicine, 9(2), pp.594-601.
- Cavender, R.K. and Fairall, M., 2009. Subcutaneous testosterone pellet implant (Testopel®) therapy for men with testosterone deficiency syndrome: A single‐site retrospective safety analysis. The Journal of Sexual Medicine, 6(11), pp.3177-3192.
- Kresch, E., Lima, T.F.N., Molina, M., Deebel, N.A., Reddy, R., Patel, M., Loloi, J., Carto, C., Nackeeran, S., Gonzalez, D.C. and Ory, J., 2023. Efficacy and safety outcomes of a compounded testosterone pellet versus a branded testosterone pellet in men with testosterone deficiency: a single-center, open-label, randomized trial. Sexual Medicine, 11(2), p.qfad007.
- Smith, R.P., Khanna, A., Coward, R.M., Rajanahally, S., Kovac, J.R., Gonzales, M.A. and Lipshultz, L.I., 2013. Factors influencing patient decisions to initiate and discontinue subcutaneous testosterone pellets (Testopel) for treatment of hypogonadism. The journal of sexual medicine, 10(9), pp.2326-2333.